

Yamate Bldg. 4F, 4-6-5 Higashi-Nakano, Nakano-ku, Tokyo 164-0003 Japan

DMP & DECCAN Executive Search-Specialist Recruitment practice focuses on recruiting full time employees (FTEs) into the permanent roles in the Life Sciences industry. Our search engagements are dedicated to the Key Opinion Leaders (KOLs), Subject Matter Experts (SMEs), and Health Care Professionals (HCPs) engaged in Clinical, Regulatory, Medical, and PV & Safety functions.
We support a range of Life Sciences industry clients such as Pharma Sponsors, Clinical Manufacturing Organizations (CMOs), Clinical Research Organizations (CROs), and domain specific technology MNCs, specializing in both Business operations and Digital cloud space (Life Sciences ICT) across departmental functions.
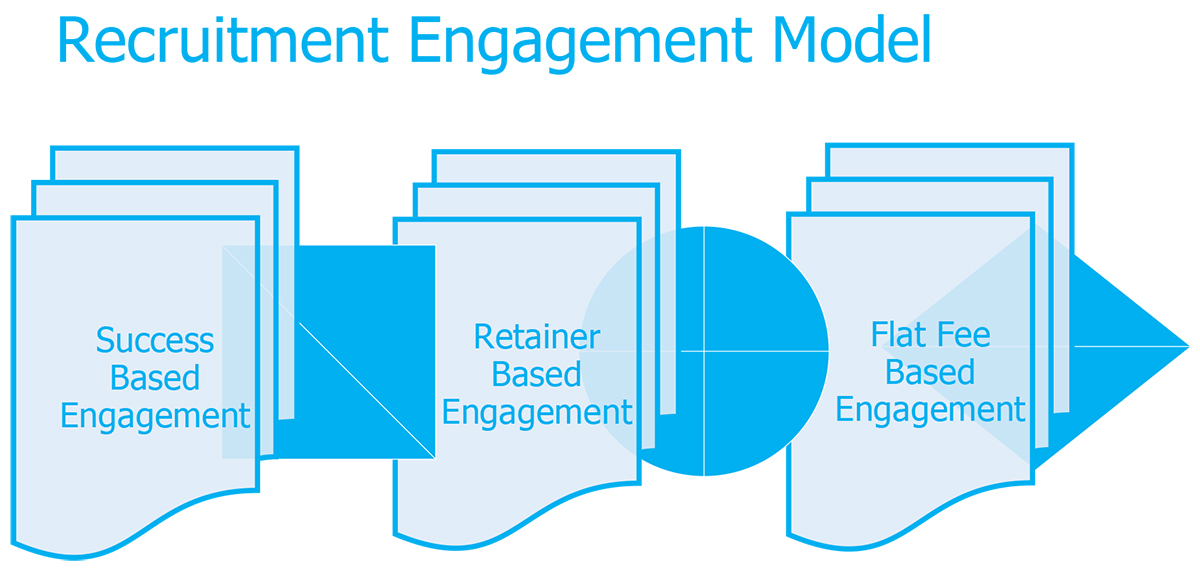
At DMP & DECCAN, we offer three different Recruitment Engagement Models (fee structures) depending on the Client’s requirements:
Success Based (Contingent Search) – by which we charge a percentage fee contingent upon the candidate is hired.
Retainer Based (Confidential Search) – by which we charge an agreed upon fee in three stages: at the start, upon acceptance of the short list and at the end of the search.
Flat Fee (Consensus Search) – an agreed upon fixed flat fee paid contingent upon the candidate is hired, intended especially for Startups.


Business Configuration | Data Migration | Technology & Deployment | Application Support | Validation, etc.
Data Entry (DE) | Quality Control (QC) | Safety Control Manager (SCM) | Project Manager (PM) | Engagement Manager (EM) | Service Delivery Manager (SDM), etc.
Clinical Research Manager (CRA/ CRM) | Clinical Field Operations and Data Management | Clinical Project Manager, etc.
Quality Compliance and Process Training | Quality Management | ISO/ GMP/ GVP Quality Auditor | QMS (Supplier Management), etc.
CMC Regulatory Affairs | APAC Regulatory Affairs | Regulatory Policy and Intelligence | Regulatory Affairs – Submission | Global Regulatory Affairs – Clinical Safety, etc.
| Medical Information & Communication | Medical Writing/ Translation | Medical Review | Pharmacovigilance & Medical Information | Medical Scientific Liaison, etc.
DMP & DECCAN GROUP K.K.
Yamate Bldg. 4F, 4-6-5 Higashi-Nakano, Nakano-ku, Tokyo
Japan- 164-0003
TEL: +81 80-9085-6658